Drug in focus: Fluconazole
Fluconazole; well known to palliative care clinicians for its use in managing severe, extensive, or treatment-resistant cases of oral candidiasis, which can significantly impact quality of life for patients. Clinical Pharmacist Karolina Powierza covers when to prescribe, interactions, side effects and more in this clinical feature on our 'Drug In Focus' for this month: Fluconazole.
This month’s drug in focus is fluconazole; a widely used triazole antifungal, well known to palliative care clinicians for its broad-spectrum activity. Its antifungal activity ranges from relatively common candidiasis to more serious systemic infections such as cryptococcosis1. As a 150mg capsule fluconazole is available without a prescription from pharmacies to help treat vaginal candidiasis2 highlighting the widespread use of fluconazole.
Fluconazole has many indications and is available in various forms and doses (50mg, 150mg, 200mg capsules, 50mg/5ml oral suspension and IV infusions). The course length can be anywhere from a once daily dose to treat vaginal candidiasis or candidal balanitis, to 400mg once daily for over 2 years to treat coccidioidomycosis3.
When to prescribe Fluconazole
For adult patients with uncomplicated oral candidiasis, it is recommended to start with topical therapy: either miconazole gel or nystatin suspension. If lesions are extensive, severe, or fail to respond to topical agents, fluconazole can be started.
Oropharyngeal candidiasis by mouth, or by intravenous infusion.
Adult
Initially 200-400mg for one dose, to be given on the first day, then 100-200mg once daily for 7–21 days (duration may be increased in severely immunocompromised patients).
Oesophageal candidiasis by mouth, or by intravenous infusion.
Adult
Initially 200-400mg for one dose, to be given on first day, then 100-200mg once daily for 14-30 days (duration may be increased in severely immunocompromised patients).3
According to NICE CKS4:
- Immunocompetent patients should receive topical nystatin or miconazole first. If thrush persists despite topical treatment, switch to oral fluconazole.
- Immunocompromised patients (for example, those with HIV, cancer, or on immunosuppressive therapy) generally warrant high-dose oral fluconazole as first-line therapy.
In the UK, storage requirements for fluconazole powder for oral suspension vary between manufacturers. Therefore, it is crucial to consult the specific Patient Information Leaflet for the most up-to-date information. For instance, the reconstituted suspension of Diflucan® (Pfizer) has a shelf life of 28 days, whereas the generic version from Genus Pharmaceuticals expires after only 14 days5,6.
National supply issues
Recently, drug shortages in the UK and more widely have become an issue that all of us face. Miconazole oral gel has not been available since May 20247 and due to the discontinuation of the Nystan® brand name, nystatin oral suspension has also been temporarily unavailable. These drug shortages have limited access to first-line treatments for oral candidiasis. In hospice settings, where oral thrush is common, fluconazole can be used as an alternative, especially for difficult-to-treat oral candidiasis or oesophageal candidiasis. While effective, fluconazole is a systemically absorbed antifungal and should be used sparingly to minimise the risk of resistance, drug interactions, and systemic side effects.
It’s important to review prescriptions for both topical and systemic antifungals. Guidelines recommend reassessing treatment after 5-7 days, and if recurrent, to seek specialist microbiological advice8.
Fluconazole in Palliative Care
Oral candidiasis is extremely common in patients receiving palliative care, most likely due to various factors including immunosuppression from treatment or disease, dry mouth or antibiotic use9. Reported prevalence of oral candidiasis in patients receiving palliative care varies in the literature; however, it appears to be between 20% and 70%10,12. Despite being common, untreated oral thrush significantly impacts quality of life, causing pain, difficulty eating, and communication problems12. While topical antifungals are first-line treatments, fluconazole’s systemic action makes it essential for managing severe, extensive, or treatment-resistant cases, particularly in immunocompromised patients or when oesophageal involvement is suspected4.
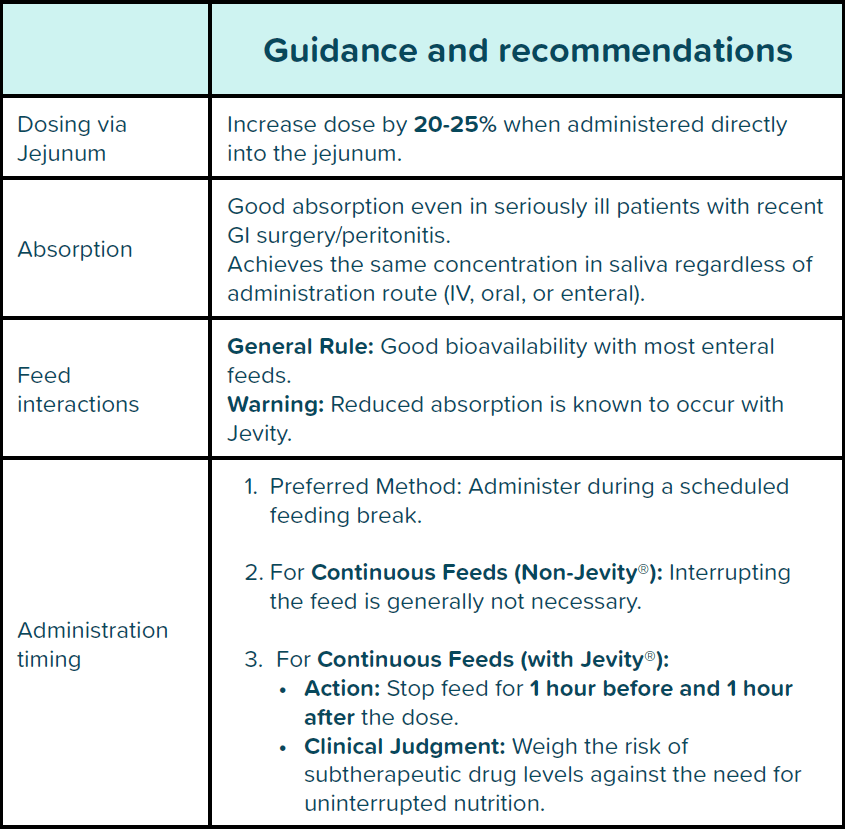
Fluconazole can be administered via enteral feeding tubes however, this requires specific dosing and administration strategies, as detailed in Table 1. below.
Interactions
It’s important to be aware that fluconazole has many interactions due to the inhibition of cytochrome P450; therefore, co-administered drugs that are metabolised through the same pathway are likely to need a dose reduction14. Specific pharmacokinetic interactions depend on the drug involved, as it’s a moderate inhibitor of CYP2C9 and CYP3A414,15. Co-administration of fluconazole with CYP3A4 inducers, such as phenytoin, phenobarbital, carbamazepine, rifampicin, and isoniazid, can accelerate the metabolism of azole antifungals, potentially leading to reduced drug levels and treatment failure. Table 2 below summarises key drug interactions relevant to palliative care.

Renal dosing and hepatic precautions
Fluconazole is approximately 80% excreted unchanged via urine; therefore, dose adjustments are often needed for patients with renal impairment.
No dose adjustment is required for single-dose therapy. For patients with impaired renal function requiring multiple doses of fluconazole, an initial loading dose of 50mg to 400mg should be administered, depending on the recommended daily dose for the specific indication. Subsequent daily dosing should then be adjusted according to the guidance outlined in the table below.

Side effects
While fluconazole is generally well tolerated, clinicians should remain alert to the following potential side effects.
Common side effects include:
- Gastrointestinal symptoms (nausea, diarrhoea, abdominal discomfort), headache, and skin rashes, which are usually mild and self-limiting17.
- Transient liver enzyme elevations are not uncommon and warrant caution in patients with pre-existing liver disease. Fluconazole has been associated with rare but serious cases of hepatic toxicity, including fatalities, most often in patients with significant underlying illness. Fortunately, hepatotoxicity is typically reversible once fluconazole is discontinued15.
- Of particular concern is fluconazole’s potential to prolong the QT interval, especially when prescribed alongside other QT-prolonging agents such as methadone, haloperidol, or certain antidepressants. This can increase the risk of life-threatening arrhythmias, including torsades de pointes. In frail or high-risk patients, baseline ECG monitoring may be warranted, especially if treatment is expected to continue beyond a few days.
- Neurological effects such as dizziness, altered taste, and, rarely, seizures have been documented, alongside haematological abnormalities like anaemia and leukopenia. Severe cutaneous reactions (e.g. Stevens-Johnson syndrome) are extremely rare but require immediate withdrawal.
In summary, while fluconazole is a valuable and generally well-tolerated antifungal, its use in palliative care should be appropriately monitored, and clinicians should be vigilant for drug interactions and organ dysfunction. With careful prescribing, its benefits often outweigh the risks in managing fungal infections in this complex population.





